Catabolism of amino acids
Summary of steps (important!)


- Generation of ammonia in periphery
- Transportation of ammonia to liver
- In periphery
- Transamination: amino acids + α-ketoglutarate ⇄ α-ketoacids + glutamate
- Transport to liver, by either
- Glutamine cycle (most common)
- Glutamate is negatively charged, it needs to get one more ammonia to form glutamine, to be able to get through membrane
- Glutamate + NH4+ + ATP → Glutamine + ADP + Pi
- Alanine cycle (Cahill cycle)
- Similar reason, glutamate gives ammonia to pyruvate to form alanine, to be able to get through membrane. This process is a reversed transamination.
- Pyruvate + glutamate ⇄ alanine + α-ketoglutarate
- Glutamine cycle (most common)
- In liver
- Convert back to glutamate, by either
- Glutaminase: glutamine + H2O → glutamate + ammonium
- Transamination: alanine + α-ketoglutarate ⇄ pyruvate + glutamate
- Release ammonia (Deamination): glutamate + NAD(P)+ + H2O ⇄ α-ketoglutarate + NH4+ + NAD(P)H + H+
- Convert back to glutamate, by either
- In periphery
- Excretion of ammonia
- Urea cycle
Transamination
- Description: transfer of an amino group from an AA to an α-ketoacid for breakdown, or to an α-ketoacid to form a nonessential AA

- E.g.
- ALT:
- AST: aspartate + α-ketoglutarate ⇄ oxalacetate + glutamate
Deamination
- Description: reaction in which an amino group from an AA is released as ammonium

Cahill cycle and Cori cycle
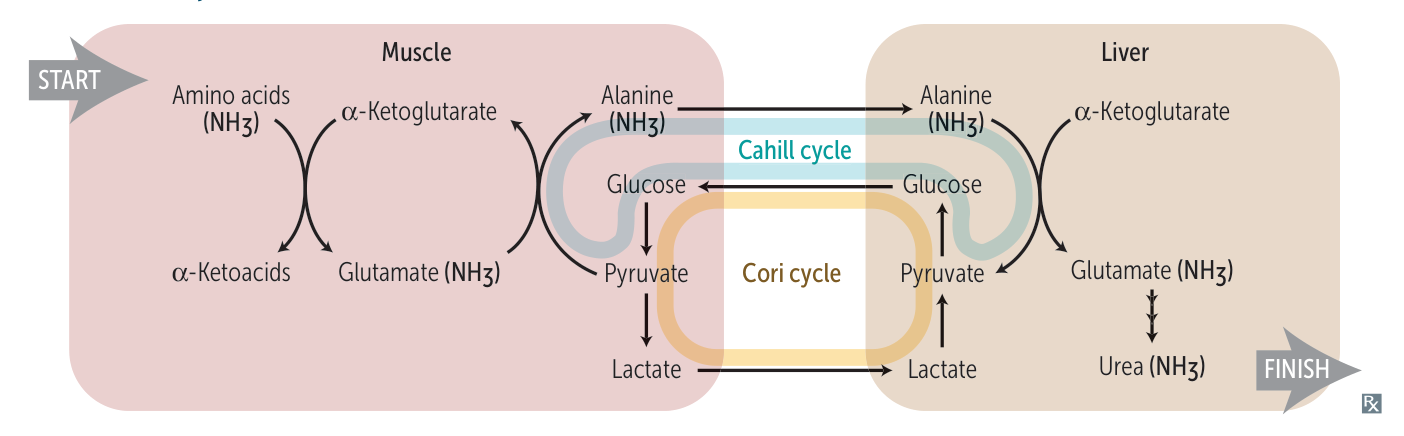
- In the liver, alanine is transaminated by alanine aminotransferase to pyruvate with the amino group being transferred to α-ketoglutarate to form glutamate. Almost all aminotransferase enzymes use α-ketoglutarate as the amino group acceptor.
- Thus, amino groups are funneled into glutamate during protein catabolism.
- Glutamate is further metabolized by the enzyme glutamate dehydrogenase, which liberates free ammonia and regenerates α-ketoglutarate.
- Ammonia then enters the urea cycle to form urea, the primary disposal form of nitrogen in humans.
- Urea subsequently enters the blood and is excreted in the urine.
Cori cycle & Cahill cycle
Lactate/alanine is transported to the liver, where it is converted into glucose. It is then transported back to the muscles for energy production.
Urea cycle
- Location: primarily occurs in the cytosol and mitochondria of liver cells and also in kidney cells


Ornithine transcarbamylase deficiency

- Definition: inherited genetic disorder characterized by the inability to excrete ammonia
- Epidemiology: most common urea cycle defect
- Inheritance: X-linked recessive (in contrast to the rest of urea cycle enzyme deficiencies which are all autosomal recessive)
- Pathophysiology
- Defect in the enzyme ornithine transcarbamylase → impaired conversion of carbamoyl phosphate and ornithine to citrulline (and phosphate) → ammonia cannot be eliminated and accumulates
- Conversion of excess carbamoyl phosphate to orotic acid occurs as part of the pyrimidine synthesis pathway
- Clinical features
- Symptoms commonly manifest in the first days of life but can develop at any age.
- Nausea, vomiting, irritability, poor feeding
- Delayed growth and cognitive impairment
- In severe cases, metabolic encephalopathy with coma and death
- Does not cause megaloblastic anemia (as opposed to orotic aciduria)
- Diagnostics
- Hyperammonemia (usually > 100 μmol/L)
- ↑ Orotic acid in urine and blood
- ↓ BUN
- ↑ Carbamoyl phosphate and ↓ citrulline in the serum
- Normal ketone and glucose levels
Digestion and absorption of dietary proteins
- Mouth: Chewing (mechanical breakdown). No chemical protein digestion.
- Stomach:
- HCl: Denatures proteins and activates pepsinogen to pepsin.
- Pepsin: Breaks proteins into smaller polypeptides.
- Small Intestine (Lumen - major digestion):
- Pancreas releases inactive enzymes (trypsinogen, chymotrypsinogen, etc.).
- Enteropeptidase (from intestinal cells) activates trypsinogen to trypsin.
- Trypsin then activates other pancreatic enzymes (chymotrypsin, carboxypeptidase).
- These enzymes break polypeptides into smaller peptides (tripeptides, dipeptides) and some free amino acids.
- Small Intestine (Brush Border & Inside Mucosal Cells - final breakdown & absorption):
- Brush border enzymes (aminopeptidases, dipeptidases, tripeptidases) on intestinal cells break small peptides into mostly free amino acids, plus some di- and tripeptides.
- Free amino acids, dipeptides, and tripeptides are absorbed into intestinal mucosal cells (enterocytes).
- Inside enterocytes: Cytosolic peptidases break down remaining di- and tripeptides into free amino acids.
- Bloodstream: Free amino acids are transported from enterocytes into the blood and travel to the liver and then to the rest of the body.